Research resources
Staff
Professional qualification Staff
Doctor 175
Pharmacist 12
Biologist 51
Biotechnologist 21
Chemist and Pharmaceutical technologist 4
Physicist 10
Psychologist 10
Research nurse 37
Technical staff 25
Study coordinator / data manager 11
Data entry 11
Oher 116
Total 483
Situation as of 31/12/2021
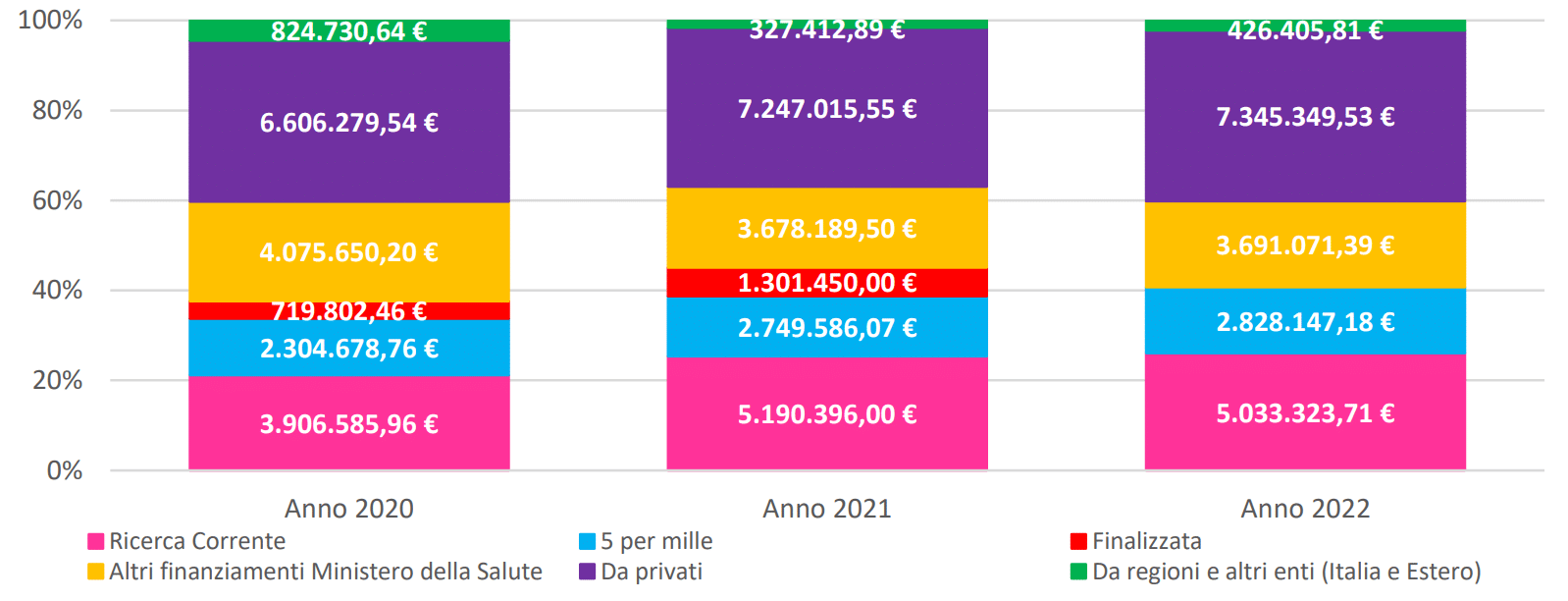
Funding
Laboratories
| Denomination | Surface (sqm) | Staff | Activity carried out | H-Index |
|---|---|---|---|---|
| Experimental Oncology | 650 | 21 | Study of the molecular mechanisms of carcinogenesis in solid and haematological tumors. Characterization of molecular alterations and biomarkers of diagnostic / prognostic / predictive value. Identification and preclinical evaluation of new therapeutic strategies. The laboratory is supported by an important animal facility of over 400 m2, which includes a sophisticated facility for in vivo molecular imaging and also a BL3 specifically dedicated to preclinical experimentation. | 282 |
| Pharmacogenetics and Pharmacogenomics | 150 | 7 | Search for polymorphisms of cellular genes that influence the response to antiblastic drugs: analysis of single genes or MASS arrays for 126 polymorphisms in 58 genes involved in the distribution, metabolism, elimination and toxicity of tumor drugs. | 62 |
| CTC laboratory | 200 | 7 | Quantification of circulating tumor cells (CTC) and tumor-derived extracellular vesicles (tdEV) and study of the genetic profile of CTC and circulating tumor DNA (ctDNA) of solid tumors; monitoring throughout the course of the disease. | 72 |
| Hereditary Cancers | 320 | 16 | Advanced molecular diagnostics (SANGER sequencing, gene panels in NGS), study and clinical management of the patient and of healthy subjects belonging to families with hereditary-familial tumors. Reference HUB center for the coordination of regional counseling centers, execution of genetic tests, registry high-risk families (6000 families monitored between Veneto and Trentino Alto Adige). | 168 |
| Oncological Virology and Prevention | 150 | 23 | Search for sequences of oncogenic viruses (HPV, EBV, HHV8, HTLV, HIV) in samples of neoplastic or preneoplastic lesions of possible viral etiology. Molecular characterization and analysis of samples containing oncogenic viruses for diagnosis, prevention and studies on the mechanisms of carcinogenesis and the natural history of infection. Research and validation of viral and cellular biomarkers useful for diagnostic purposes, predictors of the risk of neoplastic evolution and prognostics. The laboratory is supported by important BL3 and BL2 facilities. | 278 |
| Oncohematology | 100 | 15 | Analysis for diagnostic and prognostic classification and for monitoring of myelo- and lympho-proliferative diseases: 50 genetic markers available for classical and molecular cytogenetic methods – FISH, qualitative and quantitative analyzes, SANGER sequencing, NGS gene panels. | 139 |
| Cancer Immunology and Immunotherapy | 450 | 29 | Analysis of the interactions between immunity and cancer and identification of therapeutic solutions based on the stimulation of the effector components of the immune system. In particular, the activities include: – the use of experimental models in which to evaluate the effectiveness of new vaccine formulations, adoptive immunotherapy approaches and pharmacological interventions aimed at restoring the full efficiency of the immune system; – the analysis and identification of new prognostic and / or predictive immune biomarkers of response in the context of current clinical activity and ongoing trials; – immunogenetic and phenotypic-functional profiling of neoplasms of immune relevance; – the development of preclinical models for the experimentation of innovative immunotherapies. As in the case of the Experimental Oncology laboratory, part of the activities are supported by the animal facility and its instrumental equipment. | 224 |
| Anatomy and Pathological Histology | 785 | 22 | Conventional and advanced histopathological and cytopathological diagnostics with the application of immunohistochemical, immunofluorescence, chromogenic hybridization in situ techniques. Molecular diagnostics with pyrosequencing and real time PCR techniques. Search for the expression of predictive markers of response to molecularly targeted anticancer biological drugs. Histological processing and evaluation of animal tissue samples from the Immunology and Molecular Oncology Diagnostics Unit experimental research activities and biological samples from patients enrolled in clinical trials. | 63 |
| Phase 1 sample processing laboratory | 15 | 1 | Processing and storage of biological samples deriving from patients enrolled in trials. Sending of samples to the laboratory identified by the promoter of the clinical trials. | 0 |
Situation as of 30/06/2021
Last modified: 29/02/2024 11:29


